
by Beju Shah, PharmD, MBA
Key takeaways
- The Ryan Haight Act of 2008 mandated in-person evaluations for controlled substances. Although exceptions were introduced during the pandemic, they were set to end after the public health emergency but received temporary extensions.
- The DEA proposed two permanent rules emphasizing in-person exams: a 30-day supply is allowed without face-to-face consultation, but prescription refills require an in-person evaluation.
- The DEA's rules might fall short of expectations, as concerns arise from potential sidelining of pharmacists, possible loopholes like bypassing the 30-day limit, and incomplete telemedicine special registration.
You might have shared a conversation with a colleague on prescribing and telemedicine. This topic has been floating around in the hallways of my former institution of late, and it resonates with many healthcare professionals since the pandemic opened the door for patients to maintain access to care. With the end of the public health emergency, key prescribing flexibilities are at risk of phasing out. I want to share some of the main issues here as a follow-up to a previous article on temporary rules.
The impact: telehealth as a cost and quality driver in the United States
In response to the COVID-19 pandemic in 2020, the declaration of a public health emergency led to waivers and exemptions that promoted the extensive use of telehealth. It wasn't just about convenience but also a more significant issue of reducing harm as in-person visits became challenging for health systems. Also, as patients and providers adjusted to a new routine, many realized that the standard of care provided virtually could match, if not exceed, in-person consultations.
Remote care is not new
For context, remote delivery of healthcare is not a new concept. As early as November 1879, just two years after the invention of the telephone, The Lancet reported on a physician who successfully managed a child's concerning cough over a phone call.
While this scenario would not meet the expectations of providing the minimum standard of care today, remnants of this modality still exist in healthcare. For example, pharmacists providing medication therapy management (MTM) using telephone encounters are common.
Telehealth could lower spending
Today, the US has rising healthcare spending compared to other wealthy countries. Despite high spending, better outcomes are needed. With an aging population and other drivers, there is a general upward trend in spending.
As healthcare spending is a function of price and utilization, telehealth can be crucial in lowering the burden on both sides. Research has shown that telehealth can also improve the quality of care, for instance, by reducing in-person follow-up visits.
In 2022, an American Medical Association (AMA) survey revealed that nearly 85% of physician respondents currently use telehealth to care for patients, with most respondents indicating telehealth enhances patient access to care. With increasing telehealth adoption, measuring cost and quality outcomes are part of a broader trend to invest in new models of care now rather than wait for another crisis.
The problem: federal law regulations with controlled substances
The rapid growth in telemedicine has had particular challenges, especially concerning the prescription of controlled substances. Federal laws, primarily the Ryan Haight Act of 2008, were created to prevent online pharmacies from illegally dispensing controlled substances. A crucial component of this act was the mandate for an in-person medical evaluation.
While the Ryan Haight Act serves as a guardrail against bad actors, it made it more difficult for legitimate clinicians to write Schedule II - V drug prescriptions. This rigidity became evident early on, at the start of the pandemic, as in-person requirements began to limit access to crucial medications for many patients who relied on telehealth.
Exceptions to the Ryan Haight Act
For healthcare professionals, understanding the Ryan Haight Act is essential. It has seven "practice of telemedicine" exceptions to the usual in-person medical evaluation. One key exception is triggered during a public health emergency. The DEA's decision to waive the in-person exam is based on this. During these unprecedented times, regulations ease, ensuring critical treatments remain accessible.
In the book, Telehealth Success: How to Thrive in the New Age of Remote Care, doctors Aditi U. Joshi and Brandon M. Welch mention Bicycle Health as a prime example of a telemedicine company that seized these exceptions as an opportunity to bridge gaps. The virtual care clinic has played a pivotal role in providing care to vulnerable patients, especially those seeking treatment for opioid use disorder (OUD). In Alabama, when the state mandated an initial in-person visit for treatment, Bicycle Health took a stand.
They flew in physicians for several days to see hundreds of patients in a hotel conference room, ensuring no interruption in care. This initiative was highlighted in the media as the "Alabama Airdrop." Telehealth visits have proven to reduce barriers to OUD treatment, which can help prevent overdose deaths. A growing number of studies consistently demonstrate the positive outcomes of virtual care, and in many regards, it has become the forefront of healthcare.
Telemedicine challenges
While the rise of telemedicine startups has presented many advantages, it hasn't been without challenges. There have been reports of inappropriate prescribing, especially concerning ADHD and anxiety medications, raising concerns about patient safety.
This rapid adoption of telemedicine has sometimes resulted in overprescribing, disrupting the supply chain, and leading to drug shortages. With broader access to controlled substances like stimulants and opioids, there's also a heightened risk of drug diversion. This led some major pharmacy chains to implement new policies, prohibiting the dispensing of prescriptions from specific companies.
However, recent research by Epic suggests that the correlation between telehealth and overprescribing ADHD/anxiety stimulants might not be as pronounced as previously believed. While these findings highlight potential risks to the public, implementing robust clinical decision support systems and protocols can help ensure public safety.
The solution: DEA’s proposed rules after the public health emergency
With this backdrop, here is a closer look at the proposed permanent rules and their potential impacts.
Public advocacy
On March 1, 2023, the healthcare industry was abuzz when the DEA unveiled its two proposed rules concerning prescribing controlled substances through telemedicine. With 38,000 comments received, it's evident that the implications of these rules are significant for patients, prescribers, and pharmacies.
Expanding rules
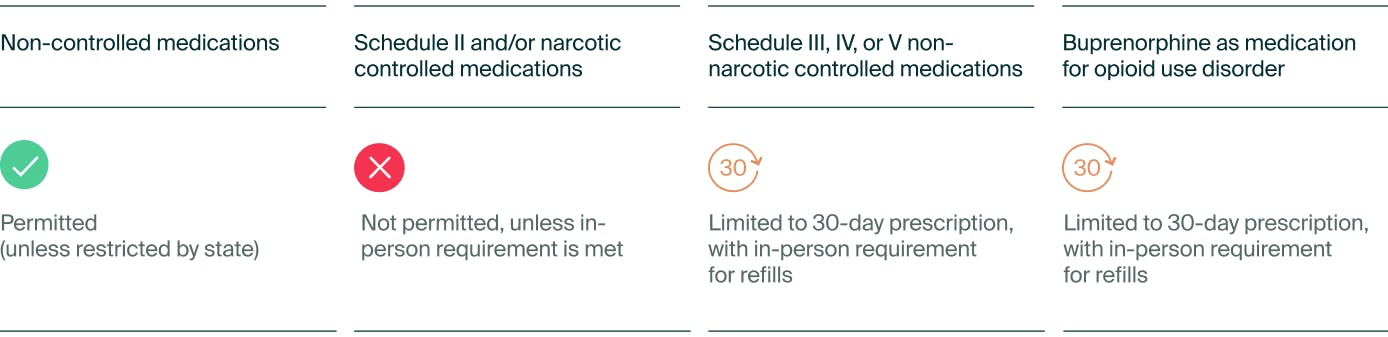
While the goal was to expand on the existing laws before the pandemic, many telehealth proponents argue that it isn't sufficient. Both rules emphasize the necessity of an in-person medical examination before obtaining prescriptions for controlled substances. The first rule focuses on prescribing Schedule III-V non-narcotic controlled substances, while the second targets Schedule III narcotics, primarily buprenorphine—a critical drug for opioid use disorder (OUD) treatment.
The DEA will offer a provision allowing telehealth practitioners to prescribe a 30-day supply of either a Schedule III-V non-narcotic controlled substance or buprenorphine for OUD without an initial in-person evaluation. However, any refill or larger prescription quantity would require an in-person consultation.
Table 1. Proposed rules for telemedicine providers prescribing online
Limitations
Beyond the 30-day supply, prescribers must comply with one of the following criteria:
- Conduct the in-person evaluation themselves.
- Be present (virtually, with audio-visual communication) when another DEA-registered provider examines the patient.
- Obtain a telemedicine referral from another provider who has already conducted an in-person assessment.
It's been a race against time, as these rules were set to be implemented following the COVID-19 public health emergency, in line with the DEA's directive from the 2008 Ryan Haight Act. However, given the narrow window—as the proposed rules were released just a few months before the end of the COVID-19 PHE—the healthcare community expressed concerns that telehealth providers might not have sufficient time to adapt, jeopardizing patient access to vital telehealth services. Fortunately, due to advocacy efforts, temporary rules were established to ensure continuity of care.
The future of prescribing: questions still remain
Operational ambiguity: The new guidelines primarily focus on prescribers, which may sideline pharmacists. Although pharmacists are responsible for verifying legitimate prescriptions under the Controlled Substances Act, the criteria for telehealth prescriptions involving controlled substances remain unclear. We might witness another influx of retail corporations refusing prescriptions for various reasons, which ultimately harms the patient.
Potential loophole: The proposed system has a potential flaw. A patient might bypass the 30-day initial prescription limitation by consulting a different telehealth practitioner for the same therapy. Although the database systems, known as prescription drug monitoring programs (PDMPs), are designed to alert clinicians about multiple prescriptions or prescribers, these systems are relatively basic in practice.
Incomplete rules: The DEA's newly proposed rule differs from what was anticipated concerning the telemedicine special registration. These rules have distinct purposes and processes stemming from different sections of the Ryan Haight Act. The special registration exception of the act needed to be completed, but it has the potential to be pivotal in enabling the legitimate prescribing of controlled substances for specific providers. The intent has always been that this registration would require more rigorous scrutiny for its application, stricter than the standard DEA registration while eliminating the need for in-person consultations. However, the DEA has previously stated that creating a special registration would be another unnecessary requirement for providers to practice.
For years, stakeholders had urged the DEA, backed by directives from the Legislative and Executive branches, to activate this registration by October 24, 2019. Yet, the rule remains inert. The DEA asserts that its recent proposal fulfills its obligation regarding special registration, but they do not specify the mandates. Although a case could be made against an additional registration process (similar to the X-waiver for buprenorphine) it could complicate the process for clinicians.
The summary: an answer lies in empowering clinicians
Undoubtedly, the DEA has introduced complex rules with technical aspects that could profoundly affect patients dependent on their medications for clinical care. When considering the expansion of telehealth regulations, it's essential to assess the added intricacy and potential bloat. Compliance can become challenging if federal and state regulations are not designed carefully, considering all stakeholders' views.
The DEA's proposed rules highlight the delicate balance between patient accessibility and safety. Although these rules strive to maintain the integrity of telehealth prescribing, the ensuing discussion unveils complex challenges that require holistic solutions. Many argue that, while there's increased flexibility compared to pre-pandemic measures, it still falls short of meeting patient needs.
As the healthcare landscape undergoes swift changes, keeping this at the heart of any legal and ethical deliberations is vital. It's paramount that regulations effectively cater to both providers and patients, ensuring smooth, safe, and accessible care.